Porous silica-encapsulated and magnetically recoverable Rh NPs: a highly efficient, stable and green catalyst for catalytic transfer hydrogenation with “slow-release” of stoichiometric hydrazine in water†
Abstract
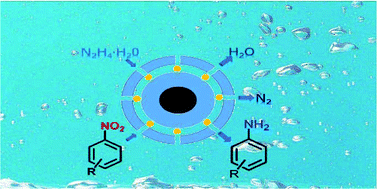
A core–shell structured nanocatalyst (Fe3O4@SiO2-NH2-RhNPs@mSiO2) that is encapsulated with porous silica has been designed and prepared for catalyzing the transfer hydrogenation of nitro compounds into corresponding amines. Rh nanoparticles serve as the activity center, and the porous silica shell plays an important role in the “slow-release” of the hydrogen source hydrazine. This reaction can be carried out smoothly in the green solvent water, and the atom economy can be improved by decreasing the amount of hydrazine hydrate used to a stoichiometric 1.5 equivalent of the substrate. Significantly, high catalytic efficiency is obtained and the turnover frequency (TOF) can be up to 4373 h−1 in the reduction of p-nitrophenol (4-NP). A kinetics study shows that the order of reaction is ∼0.5 towards 4-NP, and the apparent active energy Ea is 58.18 kJ mol−1, which also gives evidence of the high catalytic efficiency. Additionally, the excellent stability of the catalyst has been verified after 15 cycles without any loss of catalytic activity, and it is easily recovered by a magnet after reaction due to the Fe3O4 nucleus.


 Please wait while we load your content...
Please wait while we load your content...