Cranberry Proanthocyanidins – Protein complexes for macrophage activation†
Abstract
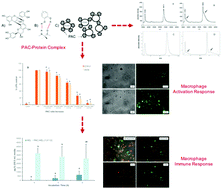
In this work we characterize the interaction of cranberry (Vaccinium macrocarpon) proanthocyanidins (PAC) with bovine serum albumin (BSA) and hen egg-white lysozyme (HEL) and determine the effects of these complexes on macrophage activation and antigen presentation. We isolated PAC from cranberry and complexed the isolated PAC with BSA and HEL. The properties of the PAC–protein complexes were studied by matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS), gel electrophoresis and zeta-potential. The effects of PAC–BSA complexes on macrophage activation were studied in RAW 264.7 macrophage like cells after treatment with lipopolysaccharide (LPS). Fluorescence microscopy was used to study the endocytosis of PAC–BSA complexes. The effects of the PAC complexes on macrophage antigen presentation were studied in an in vitro model of HEL antigen presentation by mouse peritoneal mononuclear cells to a T-cell hybridoma. The mass spectra of the PAC complexes with BSA and HEL differed from the spectra of the proteins alone by the presence of broad shoulders on the singly and doubly charged protein peaks. Complexation with PAC altered the electrophoretic mobility shift assay in native agarose gel and the electrophoretic mobility (ζ-potential) values. These results indicate that the PAC–protein complexes are stable and alter the protein structure without precipitating the protein. Fluorescence microscopy showed that the RAW 264.7 macrophages endocytosed BSA and PAC–BSA complexes in discrete vesicles that surrounded the nucleus. Macrophages treated with increasing amounts of PAC–BSA complexes had significantly reduced COX-2 and iNOS expression in response to treatment with lipopolysaccharide (LPS) in comparison to the controls. The PAC–HEL complexes modulated antigen uptake, processing and presentation in murine peritoneal macrophages. After 4 h of pre-incubation, only trace amounts of IL-2 were detected in the co-cultures treated with HEL alone, whereas the PAC–HEL complex had already reached the maximum IL-2 expression. Cranberry PAC may increase the rate of endocytosis of HEL and subsequent expression of IL-2 by the T-cell hybridomas. These results suggest that PAC–protein complexes modulate aspects of innate and acquired immune responses in macrophages.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...