Comparison of indoor air sampling and dust collection methods for fungal exposure assessment using quantitative PCR†
Abstract
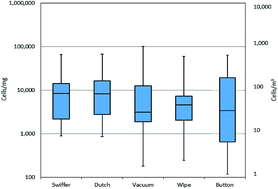
Evaluating fungal contamination indoors is complicated because of the many different sampling methods utilized. In this study, fungal contamination was evaluated using five sampling methods and four matrices for results. The five sampling methods were a 48 hour indoor air sample collected with a Button™ inhalable aerosol sampler and four types of dust samples: a vacuumed floor dust sample, newly settled dust collected for four weeks onto two types of electrostatic dust cloths (EDCs) in trays, and a wipe sample of dust from above floor surfaces. The samples were obtained in the bedrooms of asthmatic children (n = 14). Quantitative polymerase chain reaction (qPCR) was used to analyze the dust and air samples for the 36 fungal species that make up the Environmental Relative Moldiness Index (ERMI). The results from the samples were compared by four matrices: total concentration of fungal cells, concentration of fungal species associated with indoor environments, concentration of fungal species associated with outdoor environments, and ERMI values (or ERMI-like values for air samples). The ERMI values for the dust samples and the ERMI-like values for the 48 hour air samples were not significantly different. The total cell concentrations of the 36 species obtained with the four dust collection methods correlated significantly (r = 0.64–0.79, p < 0.05), with the exception of the vacuumed floor dust and newly settled dust. In addition, fungal cell concentrations of indoor associated species correlated well between all four dust sampling methods (r = 0.68–0.86, p < 0.01). No correlation was found between the fungal concentrations in the air and dust samples primarily because of differences in concentrations of Cladosporium cladosporioides Type 1 and Epicoccum nigrum. A representative type of dust sample and a 48 hour air sample might both provide useful information about fungal exposures.


 Please wait while we load your content...
Please wait while we load your content...