Coordinatively- and electronically-unsaturated square planar cobalt(iii) complexes of a pyridine dianionic pincer ligand†
Abstract
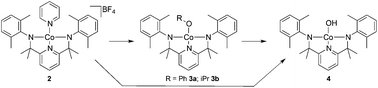
A series of low-valent Co(III) square planar complexes supported by a dianionic pincer ligand bis(arylamido)pyridine ([NNN]2−) was synthesized, including the first structurally characterized square planar hydroxide complex ([NNN]Co(OH), 4) of a 3d transition metal in the +3 oxidation state. The magnetic properties of the Co(III) complexes were highly dependent on the charge of the complex and on the coordination environment of the metal. The diamagnetic cationic complex {[NNN]Co(py)}BF4 (2) can be converted to neutral paramagnetic complexes [NNN]Co(OR) (R = Ph 3a, iPr 3b, and H 4) by a simple substitution of the ancillary pyridine ligand in 2. The double bond character between the metal and the anilido nitrogen was evident from short Co–N2,3 bond lengths in the solid-state structures, and further supported by density functional theory calculations. Complex 2 showed well-behaved redox processes at −0.93 and +0.70 V assigned to CoII/III and [NNN]2−/1− redox couples, respectively. In contrast, both complexes 3a and 4 showed some irreversibility in redox processes on either cobalt or ligands.


 Please wait while we load your content...
Please wait while we load your content...