A hydroboration route to geminal P/B frustrated Lewis pairs with a bulky secondary phosphane component and their reaction with carbon dioxide†
Abstract
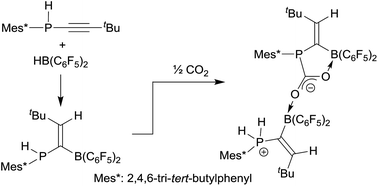
The secondary aryl-P(H) phosphanyl substituted tert-butylacetylenes 7a,b (aryl: Mes or Mes*) undergo hydroboration with [HB(C6F5)2] to give the geminal vinylidene-bridged P/B Lewis pairs 8a,b. The treatment of 8a,b with benzonitrile, N-sulfinylaniline, and phenyl isothiocyanate, respectively, gives the addition products 12a,b, 13a,b, and 14 with proton transfer from the phosphorus to the more basic nitrogen site. The reaction of the FLPs 8a,b with carbon dioxide yields a doubly boron bonded addition product. The reaction of 8b with a conjugated ynone formally proceeded by trans-1,2-hydrophosphination of the alkyne at the geminal FLP framework to give the seven-membered heterocycle 21.


 Please wait while we load your content...
Please wait while we load your content...