Half-sandwich iridium N-heterocyclic carbene anticancer complexes†
Abstract
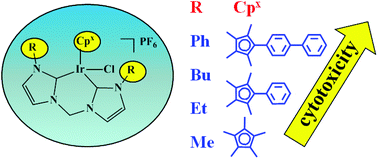
Half-sandwich pseudo-octahedral pentamethylcyclopentadienyl IrIII complexes of the type [(η5-Cpx)Ir(C^C)Cl]PF6, where Cpx is pentamethylcyclopentadienyl (Cp*), or its phenyl (Cpxph = C5Me4C6H5) or biphenyl (Cpxbiph = C5Me4C6H4C6H5) derivatives, and the C^C-chelating ligands are different N-heterocyclic carbene (NHC) ligands, have been synthesized and characterized. Three X-ray crystal structures have been determined. Except for Cp* complex 1A, the other eleven complexes 1B–4C all showed potent cytotoxicity, with IC50 values ranging from 2.9 to 46.3 μM toward HeLa human cervical cancer cells. The potency toward HeLa cells increased with additional phenyl substitution on Cp*: Cpxbiph > Cpxph > Cp*, and increased with the size of chain substitution on the C^C-ligand in the order: ph > butyl > ethyl > methyl. Complex [(η5-C5Me4C6H4C6H5)Ir(L4)Cl]PF6 (4C) displayed the highest potency, and was about 3 times more active than the clinical platinum drug cisplatin. Complexes 1A–4C all undergo hydrolysis and their kinetics was studied. DNA binding appears not to be the major mechanism of action. The ability of these iridium complexes to catalyze hydride transfer from the coenzyme NADH to NAD+ was studied. Complexes [(η5-C5Me4C6H4C6H5)Ir(L2)Cl]PF6 (2C) and [(η5-C5Me4C6H4C6H5)Ir(L3)Cl]PF6 (3C) cause cell apoptosis and arrest the cell cycle at the G1 phase and G2/M phase when HeLa cancer cells are treated with different IC50 concentrations of the complexes, and increase the amount of reactive oxygen species (ROS) dramatically, which appears to contribute to the anticancer activity. This class of organometallic Ir complexes has unusual features worthy of further exploration in the design of novel anticancer drugs.


 Please wait while we load your content...
Please wait while we load your content...