Comment on “Conformational analysis of triphenylphosphine ligands in stereogenic monometallic complexes: tools for predicting the preferred configuration of the triphenylphosphine rotor” by J. F. Costello, S. G. Davies, E. T. F. Gould and J. E. Thomson, Dalton Trans., 2015, 44, 5451†
Abstract
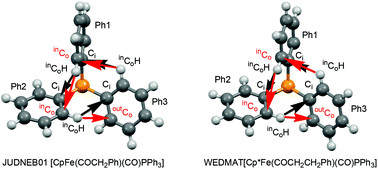
In half-sandwich compounds of the type [Cp*ML1L2PPh3] the PPh3 propeller is stabilized by attractive CH/π interactions in which Co–H bonds specifically interact with the Ci and Co atoms of neighbouring phenyl rings, as in the T-shaped benzene dimer (i/o = ipso/ortho). This stabilization was not taken into account in a recent conformational analysis based on van der Waals energy calculations and minimization of steric compression (Dalton Trans., 2015, 44, 5451–5466). It is shown that in all 116 structures discussed in this analysis the CoH–Ci/o distances fall below the sum of the van der Waals radii, establishing attractive CH/π interactions, although the short contacts could easily be avoided by phenyl rotation to relieve steric strain. In 53 of the described structures there are acyl substituents which form conformation-determining Co–H⋯O(acyl) hydrogen bonds that are not taken into account in the recent analysis. The steric-only model is not an adequate description of M–PPh3 complexes.


 Please wait while we load your content...
Please wait while we load your content...