1,8-Bis(silylamido)naphthalene complexes of magnesium and zinc synthesised through alkane elimination reactions†
Abstract
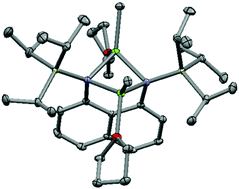
The reactions between magnesium or zinc alkyls and 1,8-bis(triorganosilyl)diaminonaphthalenes afford the 1,8-bis(triorganosilyl)diamidonaphthalene complexes with elimination of alkanes. The reaction between 1,8-C10H6(NSiMePh2H)2 and one or two equivalents of MgnBu2 affords two complexes with differing coordination environments for the magnesium; the reaction between 1,8-C10H6(NSiMePh2H)2 and MgnBu2 in a 1 : 1 ratio affords 1,8-C10H6(NSiMePh2)2{Mg(THF)2} (1), which features a single magnesium centre bridging both ligand nitrogen donors, whilst treatment of 1,8-C10H6(NSiR3H)2 (R3 = MePh2, iPr3) with two equivalents of MgnBu2 affords the bimetallic complexes 1,8-C10H6(NSiR3)2{nBuMg(THF)}2 (R3 = MePh22, R3 = iPr33), which feature four-membered Mg2N2 rings. Similarly, 1,8-C10H6(NSiiPr3)2{MeMg(THF)}2 (4) and 1,8-C10H6(NSiMePh2)2{ZnMe}2 (5) are formed through reactions with the proligands and two equivalents of MMe2 (M = Mg, Zn). The reaction between 1,8-C10H6(NSiMePh2H)2 and two equivalents of MeMgX affords the bimetallic complexes 1,8-C10H6(NSiMePh2)2(XMgOEt2)2 (X = Br 6; X = I 7). Very small amounts of [1,8-C10H6(NSiMePh2)2{IMg(OEt2)}]2 (8), formed through the coupling of two diamidonaphthalene ligands at the 4-position with concomitant dearomatisation of one of the naphthyl arene rings, were also isolated from a solution of 7.


 Please wait while we load your content...
Please wait while we load your content...