Incorporation of a platinum center in the pi-conjugated core of push–pull chromophores for nonlinear optics (NLO)†
Abstract
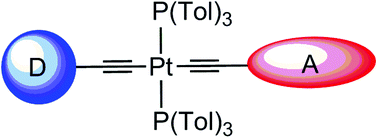
In this article, we describe the synthesis, redox characteristics, and linear and nonlinear optical (NLO) properties of seven new unsymmetrical push–pull diacetylide platinum-based complexes. These D–π-Pt–π-A complexes incorporate pyranylidene ligands as pro-aromatic donor groups (D), diazine rings as electron-withdrawing groups (A), and various aromatic fragments (styryl or thienylvinyl) as π-linkers separating the platinum diacetylide unit from the donor and the acceptor groups. This is one of the first examples of push–pull chromophores incorporating a platinum center in the π-conjugated core. The NLO properties of these complexes were compared with those of their purely organic analogues. All compounds (organic and organometallic) exhibited positive μβ values, which dramatically increased upon methylation of the pyrimidine fragment. However, this increase was even more significant in the complexes due to the presence of platinum in the π-conjugated core. The effects of the linker on the redox and spectroscopic properties of the complexes are also discussed. In addition, DFT calculations were performed in order to gain further insight into the intramolecular charge transfer (ICT) occurring through the platinum center.


 Please wait while we load your content...
Please wait while we load your content...