Brønsted acid-catalysed intramolecular hydroamination of unactivated alkenes: metal triflates as an in situ source of triflic acid†
Abstract
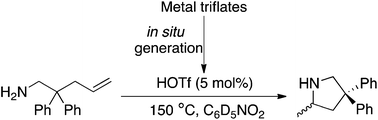
Hydroamination of alkenes or alkynes is one of the most straightforward methods to form C–N bonds and nitrogen-containing heterocycles. A simple Lewis acid Al(OTf)3 was found to be an effective precatalyst for the hydroamination of unactivated primary and secondary alkenylamines between 110 and 150 °C. Subsequent studies show that other metal triflates are also effective precatalysts for the hydroamination reactions. For metal triflate salts, mechanistic studies, including kinetics, are consistent with the in situ generation of triflic acid, which likely serves as the active catalyst.


 Please wait while we load your content...
Please wait while we load your content...