Bridgehead isomer effects in bis(phosphido)-bridged diiron hexacarbonyl proton reduction electrocatalysts†
Abstract
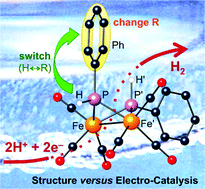
The influence of the substitution, orientation and structure of the phosphido bridges in [Fe2(CO)6(μ-PR2)2] electrocatalysts of proton reduction has been studied. The isomers e,a-[Fe2(CO)6{μ-P(Ar)H}2] (1a(Ar): Ar = Ph, 2′-methoxy-1,1′-binaphthyl (bn′)), e,e-[Fe2(CO)6{μ-P(Ar)H}2] (1b(Ar): Ar = Ph, bn′) were isolated from reactions of iron pentacarbonyl and the corresponding primary phosphine, syntheses that also afforded the phosphinidene-capped tri-iron clusters, [Fe3(CO)9(μ-CO)(μ3-Pbn′)] (2) and [Fe3(CO)9(μ3-PAr)2] (3(Ar), Ar = Ph, bn′). A ferrocenyl (Fc)-substituted dimer [Fe2(CO)6{μ:μ′-1,2-(P(CH2Fc)CH2)2C6H4}] (4), in which the two phosphido bridges are linked by an o-xylyl group, was also prepared. The molecular structures of complexes 1a(Ph), 1b(Ph), 1b(bn′), 2 and 4 were established by X-ray crystallography. All complexes have been examined as electrocatalysts for proton reduction using p-toluene sulfonic acid in tetrahydrofuran. Cyclic voltammograms of the dimers with acid exhibit two catalysis waves for proton reduction. The first wave, which appears at the potential of the primary reduction, reaches maximum current (turnover) at moderate acid concentrations and is rapidly overtaken by the second wave, which appears at more negative potential. Both of these reductive waves show an initial first order dependence on acid. The electrochemistry and electrocatalyses of the [Fe2(CO)6(μ-PR2)2] dimers show subtle variations with the nature of the bridging phosphido group(s), including the orientation of bridgehead hydrogen atoms.


 Please wait while we load your content...
Please wait while we load your content...