What regulates the catalytic activities in AGE catalysis? An answer from quantum mechanics/molecular mechanics simulations†
Abstract
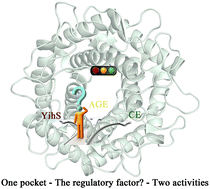
The AGE superfamily (AGEs) is made up of kinds of isomerase which are very important both physiologically and industrially. One of the most intriguing aspects of AGEs has to do with the mechanism that regulates their activities in single conserved active pocket. In order to clarify the relationship among single conserved active pocket and two activities in AGEs, results for the epimerization activity catalyzed by RaCE and the isomerization activity catalyzed by SeYihS were obtained by using QM/MM umbrella sampling simulations and 2D-FES calculations. Our results show that both of them have similar enzyme-substrate combination mode for inner pyranose ring in single conserved active pocket even though they have different substrate specificity. This means that the pathways of ring opening catalyzed by them are similar. However, one non-conserved residue (Leu183 in RaCE, Met175 in SeYihS) in the active site, which has different steric hindrance, causes a small but effective change in the direction of ring opening in stage 1. And then this change will induce a fundamentally different catalytic activity for RaCE and SeYihS in stage 2. Our results give a novel viewpoint about the regulatory mechanism between CE and YihS in AGEs, and may be helpful for further experiments of rational enzyme design based on the (α/α)6-barrel basic scaffold.

 Please wait while we load your content...
Please wait while we load your content...