What factors determine the stability of a weak protein–protein interaction in a charged aqueous droplet?†
Abstract
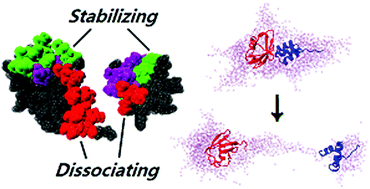
Maintaining the interface of a weak transient protein complex transferred from bulk solution to the gaseous state via evaporating droplets is a critical question in the detection of the complex association (dissociation) constant by using electrospray ionization mass spectrometry (ESI-MS). Here we explore the factors that may affect the stability of a protein–protein interaction (PPI) using atomistic molecular dynamics (MD) modelling of a complex of ubiquitin (Ub) and the ubiquitin-associated domain (UbA) (RCSB PDB code 2MRO) and a non-covalent complex of diubiquitin (RCSB PDB code 2PEA) in aqueous droplets. A general method is presented to determine the protonation states of the complexes we investigate in particular, and that of a protein in general, under various pH conditions that an evaporating droplet acquires due to its change in size. We find that the combination of high temperature and high charge states of the protein complexes may destabilize the interface by creating new interfaces instead of a direct rupture of the initial stable interface. We provide evidence that highly charged protein complexes are found in droplets that form conical extrusions of the solvent on the surface due to charge-induced instability. This distinct droplet morphology leads to a higher solvent evaporation rate that assists in transferring the complex in the gaseous state without dissociation. The conical solvent protrusions expose on the droplet surface certain amino acids that otherwise would be solvated in a droplet with the protein complex of low charge states. The new vapor-protein interface does not have a direct effect on the stability of the PPI. A common way in experiments to stabilize the protein complexes in droplets is to reduce the protonation state of the proteins. Here we find that weakly bound protein complexes even at high protonation states can be stabilized by the presence of a small number of counterions, without affecting the protonation state of the protein. Our findings may provide guiding principles in ESI-MS experiments to stabilize weak transient PPIs.


 Please wait while we load your content...
Please wait while we load your content...