Mediated water electrolysis in biphasic systems†
Abstract
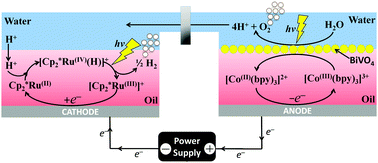
The concept of efficient electrolysis by linking photoelectrochemical biphasic H2 evolution and water oxidation processes in the cathodic and anodic compartments of an H-cell, respectively, is introduced. Overpotentials at the cathode and anode are minimised by incorporating light-driven elements into both biphasic reactions. The concepts viability is demonstrated by electrochemical H2 production from water splitting utilising a polarised water–organic interface in the cathodic compartment of a prototype H-cell. At the cathode the reduction of decamethylferrocenium cations ([Cp2*Fe(III)]+) to neutral decamethylferrocene (Cp2*Fe(II)) in 1,2-dichloroethane (DCE) solvent takes place at the solid electrode/oil interface. This electron transfer process induces the ion transfer of a proton across the immiscible water/oil interface to maintain electroneutrality in the oil phase. The oil-solubilised proton immediately reacts with Cp2*Fe(II) to form the corresponding hydride species, [Cp2*Fe(IV)(H)]+. Subsequently, [Cp2*Fe(IV)(H)]+ spontaneously undergoes a chemical reaction in the oil phase to evolve hydrogen gas (H2) and regenerate [Cp2*Fe(III)]+, whereupon this catalytic Electrochemical, Chemical, Chemical (ECC′) cycle is repeated. During biphasic electrolysis, the stability and recyclability of the [Cp2*Fe(III)]+/Cp2*Fe(II) redox couple were confirmed by chronoamperometric measurements and, furthermore, the steady-state concentration of [Cp2*Fe(III)]+ monitored in situ by UV/vis spectroscopy. Post-biphasic electrolysis, the presence of H2 in the headspace of the cathodic compartment was established by sampling with gas chromatography. The rate of the biphasic hydrogen evolution reaction (HER) was enhanced by redox electrocatalysis in the presence of floating catalytic molybdenum carbide (Mo2C) microparticles at the immiscible water/oil interface. The use of a superhydrophobic organic electrolyte salt was critical to ensure proton transfer from water to oil, and not anion transfer from oil to water, in order to maintain electroneutrality after electron transfer. The design, testing and successful optimisation of the operation of the biphasic electrolysis cell under dark conditions with Cp2*Fe(II) lays the foundation for the achievement of photo-induced biphasic water electrolysis at low overpotentials using another metallocene, decamethylrutheneocene (Cp2*Ru(II)). Critically, Cp2*Ru(II) may be recycled at a potential more positive than that of proton reduction in DCE.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...