Particle formation and growth from oxalic acid, methanesulfonic acid, trimethylamine and water: a combined experimental and theoretical study†
Abstract
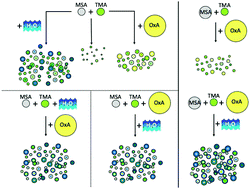
Atmospheric particles influence visibility, health and climate but the mechanisms of their formation from initial clusters and their growth to detectable particles remain largely unknown. Previous studies show that reactions of methanesulfonic acid (MSA) with ammonia and amines form particles, a process which is enhanced by water. We report here results from a combined experimental-theoretical investigation of the effect of oxalic acid (OxA) on particle formation and growth from the reaction of MSA with trimethylamine (TMA) in the absence and presence of water. The gas phase reactants were mixed in an aerosol flow reactor (1 atm, 294 K). Particle number concentrations and size distributions were measured as a function of reaction time from 0.8–12 s. The interaction of OxA with TMA with and without water does not lead to significant particle formation. When OxA is present during the reaction of MSA with TMA, there is little change (≤2 times more) in the particle number concentration but particles are larger compared to the base case of MSA with TMA alone. However, the presence of water with MSA and TMA overwhelms the effect of OxA so that OxA has no significant impact on particle number concentration or size. Results of these experiments suggest the MSA hydrate is important for particle formation and growth of the four component OxA–MSA–TMA–H2O system. These results are compared to earlier studies of the effect of OxA on the MSA–methylamine reaction and interpreted based on theoretically calculated properties of small clusters of the components.


 Please wait while we load your content...
Please wait while we load your content...