Multifunctional role of dysprosium in HfO2: stabilization of the high temperature cubic phase, and magnetic and photoluminescence properties†
Abstract
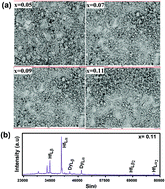
Hafnium oxide (HfO2) can exist in different crystalline structures such as monoclinic at room temperature, tetragonal at 1700 °C and cubic at 2600 °C. In the present study, nanocrystalline powders of HfO2 synthesized by a Pechini type sol–gel technique show a monoclinic phase, P21/c, at room temperature. By incorporating Dy into the HfO2 lattice, the intensity of all diffraction peaks corresponding to P21/c decreases and at a concentration of 11 at% of Dy, the monoclinic phase transforms completely to the cubic phase, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, showing a mixed phase of monoclinic and cubic at intermediate concentrations (5–9 at%) of Dy. For the first time, we have stabilized the high temperature cubic phase of HfO2 at room temperature by incorporating Dy. Selected area electron diffraction patterns confirm the monoclinic and the cubic phase as observed from the X-ray diffraction patterns. A mechanism for stabilization of the high temperature cubic phase in Hf1−xDyxO2 has been analyzed based on the substitution of dysprosium for hafnium ions and the formation of oxygen vacancies. While ferromagnetic ordering at room temperature observed in HfO2 nanoparticles is quenched after incorporating 1 at% of Dy, photoluminescence (PL) studies demonstrate excellent emissions in the blue and yellow region after exciting with UV light of wavelength 352 nm. Combining excitation and emission profiles, we have proposed a tentative energy band diagram illustrating the energetic processes taking place in Hf1−xDyxO2.
m, showing a mixed phase of monoclinic and cubic at intermediate concentrations (5–9 at%) of Dy. For the first time, we have stabilized the high temperature cubic phase of HfO2 at room temperature by incorporating Dy. Selected area electron diffraction patterns confirm the monoclinic and the cubic phase as observed from the X-ray diffraction patterns. A mechanism for stabilization of the high temperature cubic phase in Hf1−xDyxO2 has been analyzed based on the substitution of dysprosium for hafnium ions and the formation of oxygen vacancies. While ferromagnetic ordering at room temperature observed in HfO2 nanoparticles is quenched after incorporating 1 at% of Dy, photoluminescence (PL) studies demonstrate excellent emissions in the blue and yellow region after exciting with UV light of wavelength 352 nm. Combining excitation and emission profiles, we have proposed a tentative energy band diagram illustrating the energetic processes taking place in Hf1−xDyxO2.


 Please wait while we load your content...
Please wait while we load your content...