Adsorption of galloyl catechin aggregates significantly modulates membrane mechanics in the absence of biochemical cues†
Abstract
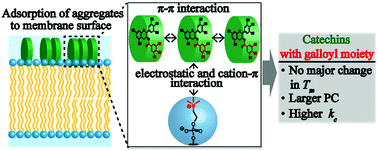
Physical interactions of four major green tea catechin derivatives with cell membrane models were systemically investigated. Catechins with the galloyl moiety caused the aggregation of small unilamellar vesicles and an increase in the surface pressure of lipid monolayers, while those without did not. Differential scanning calorimetry revealed that, in a low concentration regime (≤10 μM), catechin molecules are not significantly incorporated into the hydrophobic core of lipid membranes as substitutional impurities. Partition coefficient measurements revealed that the galloyl moiety of catechin and the cationic quaternary amine of lipids dominate the catechin–membrane interaction, which can be attributed to the combination of electrostatic and cation–π interactions. Finally, we shed light on the mechanical consequence of catechin–membrane interactions using the Fourier-transformation of the membrane fluctuation. Surprisingly, the incubation of cell-sized vesicles with 1 μM galloyl catechins, which is comparable to the level in human blood plasma after green tea consumption, significantly increased the bending stiffness of the membranes by a factor of more than 60, while those without the galloyl moiety had no detectable influence. Atomic force microscopy and circular dichroism spectroscopy suggest that the membrane stiffening is mainly attributed to the adsorption of galloyl catechin aggregates to the membrane surfaces. These results contribute to our understanding of the physical and thus the generic functions of green tea catechins in therapeutics, such as cancer prevention.


 Please wait while we load your content...
Please wait while we load your content...