Mapping gas phase dipeptide motions in the far-infrared and terahertz domain†
Abstract
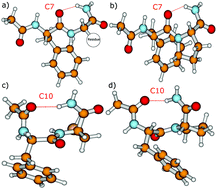
Vibrational signatures of Ac-Phe-AA-NH2 dipeptides are recorded and analysed in the far IR/THz spectral domain (100–800 cm−1, 3–24 THz), with the ‘AA’ amino acid chosen within the series ‘AA’ = Gly, Ala, Pro, Cys, Ser, Val. Phe stands for phenylalanine. IR-UV ion dip experiments are conducted on the free electron laser FELIX and combined with DFT-based molecular dynamics simulations for the calculation of the dynamical anharmonic vibrational spectra. The excellent agreements between the experimental and theoretical spectra of the Ac-Phe-AA-NH2 series allow us to make detailed and unambiguous mapping of the vibrational motions into three main domains: 700–800 cm−1 for C–H waggings, 400–700 cm−1 for N–H waggings, with a one-to-one signature per amide N–H backbone group, 0–400 cm−1 for delocalized and large amplitude collective motions over the dipeptide backbone, with backbone torsional motions arising <100 cm−1.


 Please wait while we load your content...
Please wait while we load your content...