A new architecture for high spin organics based on Baird's rule of 4n electron triplet aromatics†
Abstract
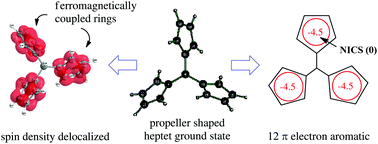
Due to the absence of open subshells (unlike transition metal compounds), stable high spin organic molecules are rare and are mostly limited to states of low multiplicity. As an alternative to high multiplicity polyradicals and polycarbenes, with their small energetic separation of different spin isomers, it is demonstrated that Baird's rule of 4n electron aromaticity in the triplet electronic state allows, in principle, the design of polycyclic high spin organics with high spin multiplicity in the electronic ground state and a large energetic separation for other spin states. Energy spacing between spin isomers is dictated here by the aromaticity or antiaromaticity of individual cycles (taking into account all π electrons), rather than by a spin Hamiltonian alone (accounting only for unpaired spin electrons). As a proof of concept, dyads of the cyclopentadienyl cation (which has been reported to possess a triplet ground state) have been computationally found to possess a quintet electronic ground state with two ferromagnetically coupled Baird aromatic rings (with SCF-GIAO NICS(0) = −4.6 and −4.4, respectively; “NICS” is “nucleus independent chemical shift”) at the CASMP2(8,10)/6-311G*//CASSCF(8,10)/6-311G* level, which is 48.3 kcal mol−1 lower in energy than the C2 open shell singlet with two antiaromatic rings (with NICS = +17.4), and 19.7 kcal mol−1 below the triplet which has one aromatic and one antiaromatic ring, with NICS = −4.8 and +45.0, respectively. Triads of the cyclopentadienyl cation in linear and branched topologies are also proposed to be ground states of maximum spin multiplicity by computations at the DFT and CCSD(T)/6-31G//UB3LYP/6-311G* levels.


 Please wait while we load your content...
Please wait while we load your content...