Comparative study of the intermolecular dynamics of imidazolium-based ionic liquids with linear and branched alkyl chains: OHD-RIKES measurements†
Abstract
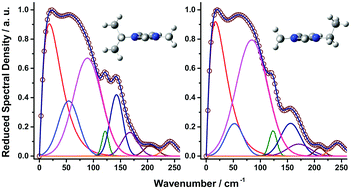
This article describes a comparative study of the low-frequency (0–450 cm−1) Kerr spectra of the branched 1-(iso-alkyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([(N − 2)mCN−1C1im][NTf2] with N = 3–7) ionic liquids (ILs) and that of the linear 1-(n-alkyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([CNC1im][NTf2] with N = 2–7) ILs. The spectra were obtained by use of femtosecond optical heterodyne-detected Raman-induced Kerr effect spectroscopy (OHD-RIKES). The intermolecular spectrum of a branched IL is similar to that of a linear IL that is of the same alkyl chain length rather than of the same number of carbon atoms in the alkyl chain. This similarity and the lack of a correlation of the first spectral moments and widths of the intermolecular spectra with chain length is mainly attributed to the increase in the dispersion contribution to the total molar cohesive energy being compensated by stretching of the ionic network due to the increasing size of the nonpolar domains, which is dependent only on the length of the alkyl chain.


 Please wait while we load your content...
Please wait while we load your content...