A combined high-temperature experimental and theoretical kinetic study of the reaction of dimethyl carbonate with OH radicals†
Abstract
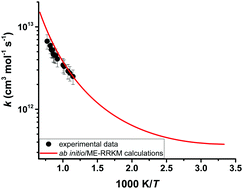
The reaction kinetics of dimethyl carbonate (DMC) and OH radicals were investigated behind reflected shock waves over the temperature range of 872–1295 K and at pressures near 1.5 atm. Reaction progress was monitored by detecting OH radicals at 306.69 nm using a UV laser absorption technique. The rate coefficients for the reaction of DMC with OH radicals were extracted using a detailed kinetic model developed by Glaude et al. (Proc. Combust. Inst. 2005, 30(1), 1111–1118). The experimental rate coefficients can be expressed in Arrhenius form as: kexpt'l = 5.15 × 1013 exp(−2710.2/T) cm3 mol−1 s−1. To explore the detailed chemistry of the DMC + OH reaction system, theoretical kinetic analyses were performed using high-level ab initio and master equation/Rice–Ramsperger–Kassel–Marcus (ME/RRKM) calculations. Geometry optimization and frequency calculations were carried out at the second-order Møller–Plesset (MP2) perturbation level of theory using Dunning's augmented correlation consistent-polarized valence double-ζ basis set (aug-cc-pVDZ). The energy was extrapolated to the complete basis set using single point calculations performed at the CCSD(T)/cc-pVXZ (where X = D, T) level of theory. For comparison purposes, additional ab initio calculations were also carried out using composite methods such as CBS-QB3, CBS-APNO, G3 and G4. Our calculations revealed that the H-abstraction reaction of DMC by OH radicals proceeds via an addition elimination mechanism in an overall exothermic process, eventually forming dimethyl carbonate radicals and H2O. Theoretical rate coefficients were found to be in excellent agreement with those determined experimentally. Rate coefficients for the DMC + OH reaction were combined with literature rate coefficients of four straight chain methyl ester + OH reactions to extract site-specific rates of H-abstraction from methyl esters by OH radicals.


 Please wait while we load your content...
Please wait while we load your content...