Supramolecular architectures based on p-cymene/ruthenium complexes functionalized with nucleobases†
Abstract
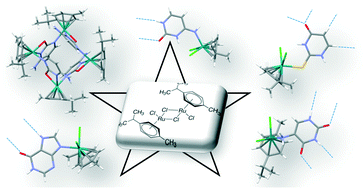
The nucleophilic attack of a series of nucleobase derivatives on the [(η6-p-cymene)RuCl(μ-Cl)]2 dinuclear entity has yielded four mononuclear complexes with the general formula [Ru(η6-p-cymene)(L)Cl2], in which L stands for cytosine (1), 2-thiouracil (2), hypoxanthine (3) and 5-aminouracil (4). Furthermore, the sieving of chlorido from compound 1, assisted by a mild base, has prompted the formation of a tetranuclear ruthenium cationic complex which crystallizes as [(η6-p-cymene)4Ru4(μ-cytosinato-κN1:κN3,O2)4](CF3SO3)4 (compound 5). The crystal structure analysis of compounds 1–4 reveals that the supramolecular packing is dominated by the self-assembly capabilities provided by the stabilized nucleobase-tautomer, which is at the same time dependent on the Ru/nucleobase coordination mode. In this regard, the assembly of the complex units is conducted through base-pairing interactions, and is also assisted by means of nucleobase/chloride hydrogen bonding. The stability of these monomeric entities in water and phosphate buffered solution is analysed by means of 1H- and 31P-NMR. The tetranuclear entity of compound 5 presents an available hydrogen-donor set (exocyclic amino group) but it lacks any available acceptor atom, which prevents the nucleobase mediated self-assembly of the complex units. Consequently, a doubly interpenetrated supramolecular network is woven by the hydrogen bonding between the triflate (CF3SO3−) anions and complex cationic entities.


 Please wait while we load your content...
Please wait while we load your content...