Catalytic substitution/cyclization sequences of O-substituted Isocyanates: synthesis of 1-alkoxybenzimidazolones and 1-alkoxy-3,4-dihydroquinazolin-2(1H)-ones†
Abstract
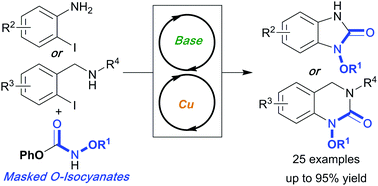
O-Substituted isocyanates (O-isocyanates) have rarely been used in organic synthesis, given their tendency to undergo side reactions (e.g., trimerization). Herein, we show that masked (blocked) O-isocyanate precursors allow one-pot or cascade reaction sequences featuring base-catalyzed substitution with 2-iodoanilines and 2-iodobenzylamines followed by copper-catalyzed cyclization, to form benzimidazolones and 3,4-dihydroquinazolin-2(1H)-ones. This work shows that O-isocyanates can serve as efficient building blocks for the synthesis of hydroxylamine-containing heterocycles.


 Please wait while we load your content...
Please wait while we load your content...