Aminoquinoline-directed, cobalt-catalyzed carbonylation of sulfonamide sp2 C–H bonds†
Abstract
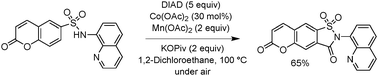
We report a method for cobalt-catalyzed, aminoquinoline-directed sp2 C–H bond carbonylation of sulfonamides. The reactions proceed in a dichloroethane solvent, and employ diisopropyl azodicarboxylate as a carbon monoxide source, Mn(OAc)2 as a cooxidant and potassium pivalate as a base. Halogen, ester, and amide functionalities are compatible with the reaction conditions. This method allows for a short and efficient synthesis of saccharin derivatives.


 Please wait while we load your content...
Please wait while we load your content...