A catalytic intramolecular nitrene insertion into a copper(i)–N-heterocyclic carbene bond yielding fused nitrogen heterocycles†
Abstract
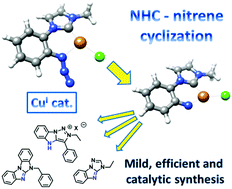
N-(2-Azidophenyl)azolium salts were easily prepared and reacted with copper(I) under conditions allowing the formation of NHC complexes. Under these conditions, the formation of benzimidazo-fused heterocycles occurred under catalytic, efficient and very mild conditions. This reaction is proposed to proceed via dinitrogen elimination and imido/nitrene–NHC cyclization.


 Please wait while we load your content...
Please wait while we load your content...