Preparation of an iron porphyrin-based polymer monolithic column via atom transfer radical polymerization for the separation of proteins and small molecules†
Abstract
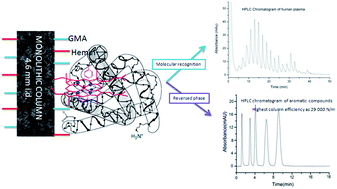
A novel iron porphyrin-based polymer monolithic column (50 × 4.6 mm i.d.) was prepared via atom transfer radical polymerization for the separation of proteins from complex bio-matrices, taking advantage of the specific absorption of iron porphyrin to protein, and the skeleton porous structure of the monolith with a high surface area of 270.3 m2 g−1. In the preparation processes, iron porphyrin and glycidyl methacrylate were used as co-monomers, ethylene dimethacrylate was used as the cross linking agent, polyethylene glycol 200 and 1,4-butanediol were used as co-porogens, N,N-dimethylformamide was used as the solvent, and CCl4 and FeCl2 were used as the initiator and catalyst, respectively. Characteristics of the optimized monolithic columns were investigated using elementary analyzer, scanning electron microscope, thermogravimetric analysis, Brunauer–Emmett–Teller nitrogen adsorption–desorption, and mercury intrusion porosimeter. The results show that the iron porphyrin-based monolithic column had an improved skeleton porous structure with micro-, meso- and macropores. The monolith was successfully used to separate protein and small molecules with high resolution and with the highest column efficiency of 29 000 plates per meter. All the results confirm that the present method is scientifically valuable for the classification of proteins as well as the separation of small molecules.

 Please wait while we load your content...
Please wait while we load your content...