Pencil graphite as an elegant electrochemical sensor for separation-free and simultaneous sensing of hypoxanthine, xanthine and uric acid in fish samples
Abstract
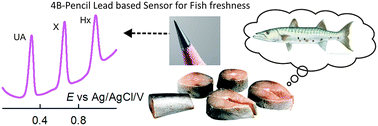
Analyzing catabolism of the fish cycle, wherein hypoxanthine (Hx) and xanthine (X) as intermediates and uric acid (UA) as the end product are formed, provides vital information about the freshness of fish. Biosensors based on xanthine oxidase have often been used for this purpose. Herein, we introduce an enzyme-free electrochemical sensor developed using an ultra-low cost 4B grade pencil graphite electrode (PGE) pre-anodized at 2 V vs. Ag/AgCl (4B-PGE*, where * means pre-anodized) as a novel electrode system for separation-free and simultaneous differential pulse voltammetric (DPV) detection of three purine bases, Hx, X and UA, in a pH 7 phosphate buffer solution. Stable and well-defined peaks at 0.95, 0.65 and 0.3 V vs. Ag/AgCl were noticed upon electrochemical oxidation of Hx, X and UA respectively at the 4B-PGE*. The 4B-PGE* is found to show about a twenty five times higher electrochemical response than the 4B-PGE (non-preanodized) for the purine oxidations. Under optimal DPV conditions, the 4B-PGE* showed linear calibration plots with current linearities in the ranges 6–30 μM, 8–36 μM and 3–21 μM with current sensitivities of 0.921 μA μM−1, 1.742 μA μM−1 and 0.499 μA μM−1 for Hx, X and UA respectively. Ten consecutive detections of 10 μM Hx, X and UA showed a relative standard deviation (RSD) of 2.14%, 4.95% and 0.32%, respectively. In order to validate the analytical approach, separation-free and simultaneous electrochemical detection of Hx, X and UA in five freshly dead fish samples, stored at different temperatures and for different storage times, was successfully demonstrated.


 Please wait while we load your content...
Please wait while we load your content...