In situ quantification and evaluation of ClO−/H2S homeostasis in inflammatory gastric tissue by applying a rationally designed dual-response fluorescence probe featuring a novel H+-activated mechanism†
Abstract
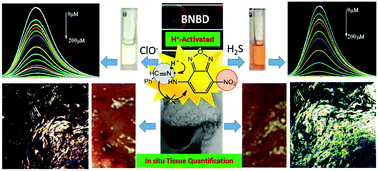
Homeostasis of ClO−/H2S plays a crucial role in the damage and repair of gastric tissue, but has rarely been investigated due to the challenge of in situ analysis in the highly acidic gastric environment. Herein, we designed a new H+-activated optical mechanism, involving controllable photoinduced electron transfer (PET) and switch of electron push–pull (SEPP), to develop the simple yet multifunctional probe (Z)-4-(2-benzylidenehydrazinyl)-7-nitrobenzo[c][1,2,5]oxadiazole (BNBD). First, the BNBD probe (Off) was protonated by the highly acidic media to trigger strong fluorescence (On). Then, the analytes ClO− and H2S reacted with the protonated BNBD, leading to ultrasensitive (ClO−: 2.7 nM and H2S: 6.9 nM) fluorescence quenching via the rapid oxidation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (50 s) and nitro reduction (10 s), respectively. With the logical discrimination by absorbance/colour (ClO−: 300 nm/colorless and H2S: 400 nm/orange), a strategy for the in situ quantification of ClO−/H2S in gastric mucosa and juice was developed. For the first time, the in situ quantitative monitoring of endogenous H2S and ClO−/H2S homeostasis as well as the pathologic manifestation in gastric mucosa were realized, thus overcoming the challenge of ClO−/H2S analysis under highly acidic conditions and enabling the in situ tissue quantification of ClO−/H2S. In combination with the assessment of mucosal damage, this study confirms the injurious/rehabilitative effects of ClO−/H2S on gastric mucosa (at 50–90 μm depth), which may facilitate the auxiliary diagnosis of stomach diseases induced by oxidative stress.
N (50 s) and nitro reduction (10 s), respectively. With the logical discrimination by absorbance/colour (ClO−: 300 nm/colorless and H2S: 400 nm/orange), a strategy for the in situ quantification of ClO−/H2S in gastric mucosa and juice was developed. For the first time, the in situ quantitative monitoring of endogenous H2S and ClO−/H2S homeostasis as well as the pathologic manifestation in gastric mucosa were realized, thus overcoming the challenge of ClO−/H2S analysis under highly acidic conditions and enabling the in situ tissue quantification of ClO−/H2S. In combination with the assessment of mucosal damage, this study confirms the injurious/rehabilitative effects of ClO−/H2S on gastric mucosa (at 50–90 μm depth), which may facilitate the auxiliary diagnosis of stomach diseases induced by oxidative stress.


 Please wait while we load your content...
Please wait while we load your content...