Structure advantage and peroxidase activity enhancement of deuterohemin-peptide–inorganic hybrid flowers†
Abstract
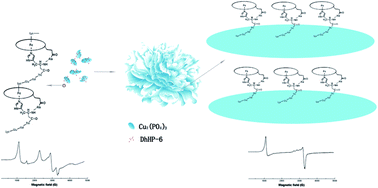
This work reports a facile method for hybridizing deuterohemin-peptide (DhHP-6) with copper phosphate to form deuterohemin-peptide–inorganic hybrid flowers (DhHP-6–Cu3(PO4)2) by self-assembly. The DhHP-6–Cu3(PO4)2 flowers have been characterized by scanning electron microscopy, Fourier transform infrared spectroscopy and solid state UV-vis. In the assembly process, the DhHP-6 peptides are fixed on Cu3(PO4)2 through the coordination of the end amino acids (Lys6) with copper(II) centers. The rearrangement of Lys6 prevents DhHP-6 aggregation. Hence, DhHP-6–Cu3(PO4)2 flowers exhibit a nearly 300% enhancement of peroxidase-like activity in comparison with free DhHP-6 in solution. Meanwhile, the DhHP-6–Cu3(PO4)2 flowers show stronger affinity towards 3,3,5,5-tetramethylbenzidine (TMB) and H2O2 than free DhHP-6 and horseradish peroxidase (HRP). In addition, EPR results provide direct evidences for the mechanisms of DhHP-6–Cu3(PO4)2 flower growth and activity enhancement. Furthermore, the sample presents excellent reusability and storage stability.

 Please wait while we load your content...
Please wait while we load your content...