Novel derivatives of 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic acid: synthesis, electrochemical and optical properties†
Abstract
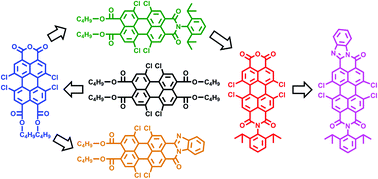
A family of novel unsymmetrical “peri”-substituted perylene-3,4,9,10-tetracarboxylic acid derivatives (5–10), with 1,6,7,12-tetrachloro-substituents at the bay-positions, has been synthesized. Subsequently, their redox and optical properties have been explored with the intent of unveiling opto-electronic characteristics of these newly synthesized compounds. To synthesize these new compounds, pure 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic tetra-n-butylester (4) has been employed as the precursor and the structural modifications have been carried out exclusively at the “peri” positions in an efficient manner. The two synthons prepared in this work, 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic di-n-butylester monoanhydride (5) and 1,6,7,12-tetrachloroperylene-3,4,9,10-tetracarboxylic monoimide monoanhydride (8), are extremely valuable and versatile starting materials as they possess free anhydride functionality at the “peri” position in addition to the 1,6,7,12-tetrachloro-bay-substituents. Finally, the conventional methodology for the synthesis of 1,6,7,12-tetraphenoxy-bay-functionalized perylene bisimides and perylene bisbenzimidazoles has been modified to make it faster and more convenient.

 Please wait while we load your content...
Please wait while we load your content...