Safety evaluation of repeated intravenous infusion of sinoporphyrin with and without PDT in rats†
Abstract
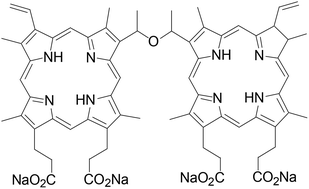
Photodynamic therapy (PDT) is a promising antineoplastic modality in the oncology field. We assessed the safety of repeated intravenous administrations of sinoporphyrin, a porphyrin derivative, with and without illumination in rats. Toxicokinetic studies of single and multiple administrations of sinoporphyrin were also carried out. Sprague-Dawley rats were randomly assigned to the dark-toxicity and PDT groups. Animals in the dark toxicity group received an i.v. infusion of sinoporphyrin at 3 doses: 2 mg kg−1, 6 mg kg−1, and 18 mg kg−1. The PDT group included 2 doses of sinoporphyrin (2 mg kg−1 and 18 mg kg−1), and the rats received 60 J of 630 nm laser illumination 24 h after photosensitizer infusion. The treatments were repeated every 7 days for 5 cycles and were followed by a 14-day recovery period. Systematic analyses were conducted at the end of treatment and recovery periods. Blood samples were obtained 5 min, 30 min, 2 h, 8 h, 24 h, 48 h, 72 h, and 96 h after the first and fifth treatments for toxicokinetic studies. Sinoporphyrin-PDT led to the death of one out of 270 rats; the dead animal had been treated with 18 mg kg−1 sinoporphyrin and died at the end of the fifth PDT treatment. Liver injury, the primary toxicity observed in the study, was identified using biochemical tests, necropsy, and histopathology. Elevated white blood cell and neutrophil counts were found in the rats in both the dark toxicity and PDT groups. Skin lesions at the illumination site were obvious in the PDT group. Pigment deposits were detected in multiple organs such as the liver, spleen, lymph nodes, and ovaries in the 6 mg kg−1 and 18 mg kg−1 groups. No other abnormalities were observed. The toxicokinetic parameters of single and multiple sinoporphyrin administrations were calculated and compared. Repeated sinoporphyrin administrations both alone and in combination with laser illumination were tolerable, and all toxicities were transient. The no observed adverse effect level (NOAEL) for repeated sinoporphyrin administration and sinoporphyrin-PDT was 6 mg kg−1 and 2 mg kg−1, respectively. Further studies are warranted.

 Please wait while we load your content...
Please wait while we load your content...