Competing amination and C–H arylation pathways in Pd/xantphos-catalyzed transformations of binaphthyl triflates: switchable routes to chiral amines and helicene derivatives†
Abstract
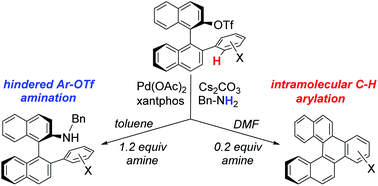
A Pd(OAc)2/xantphos catalyst system was found effective for benzylaminations of binaphthyl 2-triflates bearing a variety of alkyl, benzyl, and substituted phenyl substituents at the 2′-position. With 2′-aryl substituents, an intramolecular Pd-catalyzed C–H arylation was observed as a competing side reaction under some conditions. By adjusting the solvent and quantity of the amine, the reaction was optimized to favor either the amination or the C–H arylation pathway, affording two distinct and potentially useful sets of products. The amines represent tunable chiral ligand precursors, while the C–H arylation pathway affords a series of benzofused [5]helicene derivatives. Kinetic studies and activation parameters for the C–H arylation pathway, supported by DFT calculations, are consistent with a concerted metalation–deprotonation (CMD) mechanism involving a Pd-bound carbonate as the base. Xantphos is proposed to facilitate the turnover-limiting inner-sphere CMD step by acting as a hemilabile ligand, while its wide bite angle engenders a low reductive elimination barrier.

 Please wait while we load your content...
Please wait while we load your content...