Anthranilic acid-containing cyclic tetrapeptides: at the crossroads of conformational rigidity and synthetic accessibility†
Abstract
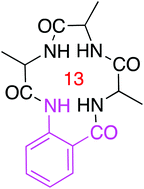
Each amino acid in a peptide contributes three atom units to main-chains, hence natural cyclic peptides can be 9, 12, 15, …. i.e. 3n membered-rings, where n is the number of amino acids. Cyclic peptides that are 9 or 12-membered ring compounds tend to be hard to prepare because of strain, while their one amino acid homologs (15-membered cyclic pentapeptides) are not conformationally homogeneous unless constrained by strategically placed proline or D-amino acid residues. We hypothesized that replacing one genetically encoded amino acid in a cyclic tetrapeptide with a rigid β-amino acid would render peptidomimetic designs that rest at a useful crossroads between synthetic accessibility and conformational rigidity. Thus this research explored non-proline containing 13-membered ring peptides 1 featuring one anthranilic acid (Anth) residue. Twelve cyclic peptides of this type were prepared, and in doing so the viability of both solution- and solid-phase methods was demonstrated. The library produced contained a complete set of four diastereoisomers of the sequence 1aaf (i.e. cyclo-AlaAlaPheAnth). Without exception, these four diastereoisomers each adopted one predominant conformation in solution; basically these conformations feature amide N–H vectors puckering above and below the equatorial plane, and approximately oriented their N–![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) atoms towards the polar axis. Moreover, the shapes of these conformers varied in a logical and predictable way (NOE, temperature coefficient, D/H exchange, circular dichroism). Comparisons were made of the side-chain orientations presented by compounds 1aaa in solution with ideal secondary structures and protein–protein interaction interfaces. Various 1aaa stereoisomers in solution present side-chains in similar orientations to regular and inverse γ-turns, and to the most common β-turns (types I and II). Consistent with this, compounds 1aaa have a tendency to mimic various turns and bends at protein–protein interfaces. Finally, proteolytic- and hydrolytic stabilities of the compounds at different pHs indicate they are robust relative to related linear peptides, and rates of permeability through an artificial membrane indicate their structures are conducive to cell permeability.
atoms towards the polar axis. Moreover, the shapes of these conformers varied in a logical and predictable way (NOE, temperature coefficient, D/H exchange, circular dichroism). Comparisons were made of the side-chain orientations presented by compounds 1aaa in solution with ideal secondary structures and protein–protein interaction interfaces. Various 1aaa stereoisomers in solution present side-chains in similar orientations to regular and inverse γ-turns, and to the most common β-turns (types I and II). Consistent with this, compounds 1aaa have a tendency to mimic various turns and bends at protein–protein interfaces. Finally, proteolytic- and hydrolytic stabilities of the compounds at different pHs indicate they are robust relative to related linear peptides, and rates of permeability through an artificial membrane indicate their structures are conducive to cell permeability.

 Please wait while we load your content...
Please wait while we load your content...