Photoswitchable anticancer activity via trans–cis isomerization of a combretastatin A-4 analog†
Abstract
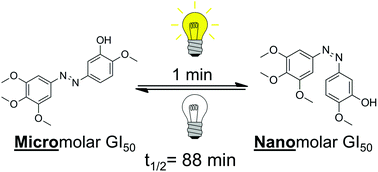
Combretastatin A-4 (CA4) is highly potent anticancer drug that acts as an inhibitor of tubulin polymerization. The core of the CA4 structure contains a cis-stilbene, and it is known that the trans isomer is significantly less potent. We prepared an azobenzene analog of CA4 (Azo-CA4) that shows 13–35 fold enhancement in potency upon illumination. EC50 values in the light were in the mid nM range. Due to its ability to thermally revert to less toxic trans form, Azo-CA4 also has the ability to automatically turn its activity off with time. Azo-CA4 is less potent than CA-4 because it degrades in the presence of glutathione as evidenced by UV-Vis spectroscopy and ESI-MS. Nevertheless, Azo-CA4 represents a promising strategy for switchable potency for treatment of cancer.

 Please wait while we load your content...
Please wait while we load your content...