Four cobalt(ii) complexes based on a new tricarboxylate with a naphthalene ring and different N-containing ligands: synthesis, crystal structures and magnetic properties†
Abstract
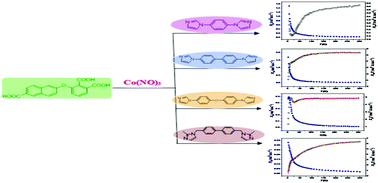
Four novel cobalt(II) complexes {[Co3(L)2(dib)3(H2O)2]·2H2O}n (1), {[Co3(L)2(4,4′-bibp)3(H2O)4]·2H2O}n (2), {[Co(HL)(bidpe)]·H2O}n (3) and {[Co2(L)(bib)0.5(μ3-OH)]·2H2O}n (4) have been prepared by employing Co(II) nitrate, a tricarboxylate ligand (H3L) and different N-containing ligands under hydrothermal conditions, where H3L = 3-((6-carboxynaphthalen-2-yl)oxy)phthalic acid, dib = 1,4-di(1H-imidazol-1-yl)benzene, 4,4′-bibp = 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl, bidpe = 1,1′-(oxybis(4,1-phenylene))bis(1H-imidazole), bib = 4,4′-bis((2-methyl-1H-imidazol-1-yl)methyl)-1,1′-biphenyl. Single crystal X-ray analysis reveals that complex 1 exhibits an infinite 2D framework formed by the L3− and dib ligands, complex 2 displays a 3D supramolecular architecture linked by hydrogen bonds, complex 3 features a 1D chain in which lattice water molecules locate with the help of hydrogen bonds, complex 4 is a homochiral 3D network with right-handed 43 and 21 helices. The phase purity, IR spectra and thermal stabilities of 1–4 have been investigated. Additionally, the magnetic behaviors for 1–4 have also been studied, revealing antiferromagnetic interactions between Co(II) ions in them.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...