Design, synthesis and anti-proliferative activities of novel 7′-O-substituted schisantherin A derivatives†‡
Abstract
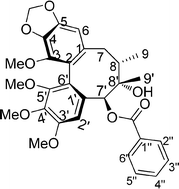
A series of schisantherin A (1) derivatives were efficiently synthesized utilizing Yamaguchi esterification (2,4,6-trichlorobenzoyl chloride, Et3N, THF, DMAP, toluene) at the C-7′ position of the schisantherin A core. The synthesized derivatives were evaluated for their anti-cancer activities against SIHA, PANC 1, MDA-MB-231, IMR-32, DU-145 and A549 cancer cell lines using sulforhodamine B assay. Within the new series tested, compound 29 displayed the most promising cytotoxic effect against the human cervical cancer cell line (SIHA) with a GI50 value of <0.01 μM, which is comparable to that of the standard drug, doxorubicin. Mechanism of action studies validated that 29 functions as a microtubule inhibitor. Additionally, several of the other analogues exhibited potent activity against the tested cell lines. Based on the results obtained, structure–activity relationships (SARs) were established and a correlation between the activities was also observed and discussed.

 Please wait while we load your content...
Please wait while we load your content...