Assessing the ligand selectivity of sphingosine kinases using molecular dynamics and MM-PBSA binding free energy calculations†
Abstract
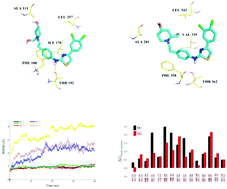
The dynamic balance of sphingolipids plays a crucial role in diverse biological processes such as mitogenesis, cell migration and angiogenesis. Sphingosine kinases (SKs) including SK1 and SK2 phosphorylate sphingosine to sphingosine 1-phosphate (S1P), and control the critical balance. SK1 overexpression was reported to increase cell survival and proliferation. Although several SK1 selective inhibitors have been reported, detailed analysis toward their selectivity to understand the molecular mechanism has not been performed to our knowledge. Herein, the crystal structure of SK1 and a homology model of SK2 were used to dock five inhibitors (1, 2, 3, 4 and 5). Protein–ligand complexes were then subjected to a molecular dynamics study and MM-PBSA binding free energy calculations. By analyzing the binding model of these inhibitors, we found that residues ILE170, PHE188 and THR192 in SK1 significantly contribute a favorable binding energy to the selectivity.

 Please wait while we load your content...
Please wait while we load your content...