Microfluidic co-culture platform to quantify chemotaxis of primary stem cells†
Abstract
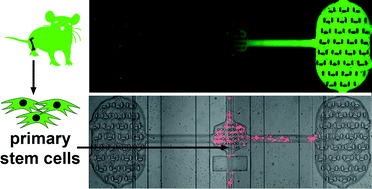
Functional analysis of primary tissue-specific stem cells is hampered by their rarity. Here we describe a greatly miniaturized microfluidic device for the multiplexed, quantitative analysis of the chemotactic properties of primary, bone marrow-derived mesenchymal stem cells (MSC). The device was integrated within a fully customized platform that both increased the viability of stem cells ex vivo and simplified manipulation during multidimensional acquisition. Since primary stem cells can be isolated only in limited number, we optimized the design for efficient cell trapping from low volume and low concentration cell suspensions. Using nanoliter volumes and automated microfluidic controls for pulsed medium supply, our platform is able to create stable gradients of chemoattractant secreted from mammalian producer cells within the device, as was visualized by a secreted NeonGreen fluorescent reporter. The design was functionally validated by a CXCL/CXCR ligand/receptor combination resulting in preferential migration of primary, non-passaged MSC. Stable gradient formation prolonged assay duration and resulted in enhanced response rates for slowly migrating stem cells. Time-lapse video microscopy facilitated determining a number of migratory properties based on single cell analysis. Jackknife-resampling revealed that our assay requires only 120 cells to obtain statistically significant results, enabling new approaches in the research on rare primary stem cells. Compartmentalization of the device not only facilitated such quantitative measurements but will also permit future, high-throughput functional screens.

 Please wait while we load your content...
Please wait while we load your content...