Raising the N-aryl fluoride content in unsymmetrical diaryliminoacenaphthylenes as a route to highly active nickel(ii) catalysts in ethylene polymerization†
Abstract
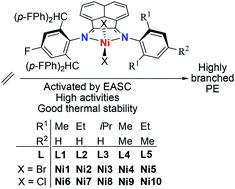
Five examples of selectively fluorinated unsymmetrical diiminoacenaphthylenes, 1-[2,6-{(4-FC6H4)2CH}2-4-FC6H4N]-2-(ArN) C2C10H6 (Ar = 2,6-Me2C6H3L1, 2,6-Et2C6H3L2, 2,6-iPr2C6H3L3, 2,4,6-Me3C6H2L4, 2,6-Et2-4-MeC6H2L5), have been synthesized and used to prepare their corresponding nickel(II) halide complexes, LNiBr2 (Ni1–Ni5) and LNiCl2 (Ni6–Ni10). Both 1H and 19F NMR spectroscopy techniques have been employed to characterize paramagnetic Ni1–Ni10; an inequivalent fluorine environment is a feature of the tetrahedral complexes in solution. Upon activation with relatively low ratios (ca. 600 equiv.) of ethylaluminum sesquichloride (Et3Al2Cl2, EASC), all the nickel complexes displayed high activities toward ethylene polymerization at 30 °C with precatalyst Ni4 the standout performer at 2.20 × 107 g of PE per mol of Ni per h, producing highly branched polyethylenes. In comparison with related diiminoacenaphthylene-nickel catalysts, these current systems, incorporating a high fluorine content on one N-aryl group, display superior productivity. In addition, the molecular structures of Ni2 and Ni4 are reported and the active catalyst is probed using 19F NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...