Tridentate Lewis acids with phenyl substituted 1,3,5-trisilacyclohexane backbones†
Abstract
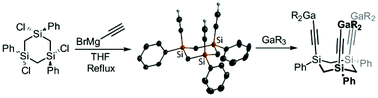
The 1,3,5-trisilacyclohexane skeleton [CH2Si(Ph)(C2H)]3 equipped with three ethynyl groups was synthesised and functionalised to afford tridentate Lewis acids with directed functions. Hydroboration with HB(C6F5)2 yielded the ethenylborane {CH2Si(Ph)[C2H2B(C6F5)2]}3, while metalation with gallium organyls afforded {CH2Si(Ph)[C2Ga(R)2]}3 (R = Me, Et). The new compounds were identified by elemental analyses, multi-nuclear NMR spectroscopy and IR spectroscopy. Crystal structures were obtained for all-cis-[CH2Si(Ph)(OMe)]3, cis–trans-[CH2Si(Ph)(Cl)]3, all-cis-[CH2Si(Ph)(H)]3 and all-cis-[CH2Si(Ph)(C2H)]3.

 Please wait while we load your content...
Please wait while we load your content...