Chemistry in confined space: a strategy for selective oxidation of hydrocarbons with high catalytic efficiencies and conversion yields under ambient conditions†
Abstract
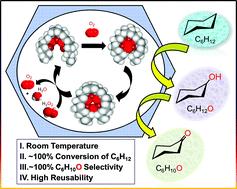
Selective catalytic oxidation of hydrocarbons is challenging. Here, we show how this chemistry could be accomplished for cyclohexane (C6H12) at room temperature with good turnover numbers, excellent catalytic efficiencies, high conversion yields and product selectivity, when the catalysis is mediated by a tricopper cluster complex immobilized in the nanochannels of mesoporous silica nanoparticles. The CuICuICuI tricopper cluster is activated by dioxygen (O2) to mediate the hydrocarbon oxidation to cyclohexanol (C6H12O) and cyclohexanone (C6H10O) by a direct O-atom transfer mechanism and the turnover of the catalyst is driven by hydrogen peroxide (H2O2). The desired product is obtained by simply varying the experimental conditions. In the case of limiting H2O2, the catalytic efficiency can reach 96%. When H2O2 is in large excess, the conversion of C6H12 and selectivity to C6H10O can reach close to 100%. The nano-confined catalytic system leads to higher solubility of O2 and thus to higher activity. The heterogeneous catalyst is robust and reusable after many cycles.


 Please wait while we load your content...
Please wait while we load your content...