Novel poly(2-oxazoline)s with pendant l-prolinamide moieties as efficient organocatalysts for direct asymmetric aldol reaction†
Abstract
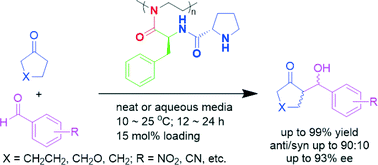
Poly(2-oxazoline)-supported bifunctional organocatalysts have been prepared through a bottom-up protocol, which involves synthesis of well-defined poly(2-oxazoline) precursors bearing amino groups in the side-chains followed by amide coupling with N-Boc-L-proline then deprotection. The resultant L-prolinamido-functionalized polymers have proven to be significantly more active than their monomeric counterparts for the aldolisation of cyclic ketones with several substituted benzaldehydes under neat conditions. By using 15 mol% of the polymer as a catalyst, the direct aldol reaction products were isolated in high yields and with good diastereo- and enantioselectivity. Based on circular dichroism spectrum analysis, the enhancement in catalytic activity is probably related to the conformational changes of the pseudo-peptide scaffold of poly(2-oxazoline)s. In addition, these soluble polymeric catalysts can be recovered and reused by precipitation in ether for five catalytic cycles without significantly diminishing their efficiency.

 Please wait while we load your content...
Please wait while we load your content...