Continuous flow Negishi cross-couplings employing silica-supported Pd-PEPPSI–IPr precatalyst†
Abstract
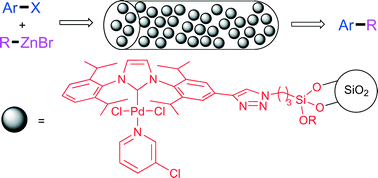
The synthesis of a triethoxysilyl functionalised Pd-PEPPSI–IPr complex prepared via azide–alkyne cycloaddition is described. The complex was immobilised onto silica gel and applied as a heterogeneous catalyst in the Negishi reaction. The catalyst was active in both batch and continuous flow operation and was particularly effective for the coupling of heteroaryl chlorides. Long-term continuous flow experiments demonstrated good catalyst activity over fifteen hours.
- This article is part of the themed collection: Catalysis in Flow Chemistry

 Please wait while we load your content...
Please wait while we load your content...