Epoxidation using molecular oxygen in flow: facts and questions on the mechanism of the Mukaiyama epoxidation
Abstract
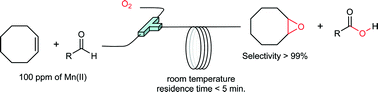
The Mukaiyama epoxidation was studied using a segmented flow reactor. Full conversion of cis-cyclooctene (0.67 M) with high selectivity (>99%) towards cyclooctene oxide was reached in 5 minutes using 2-ethylhexanal (2 M) as a sacrificial co-reductant, 100 ppm of Mn(II) as catalyst and 5 bar of oxygen. To gain further insights into the Mukaiyama reaction we have investigated the influence of the cis-cyclooctene/2-ethylhexanal ratio and cis-cyclooctene concentration. It was shown that after an induction period during which time aldehyde was oxidised faster than the alkene, the theoretical stoichiometry of the Mukaiyama reaction (1 mole of epoxide per mole of oxidised aldehyde) was observed. Then, different metal catalysts and catalyst loading were evaluated. The best results were obtained with Mn(II). We can suspect that the differences in productivity described in the literature for Mukaiyama epoxidation reaction could be explained by oxygen mass transfer resistance rather than catalytic efficiency. Surprisingly the selectivity of 2-ethylhexanal oxidation toward 2-ethylhexanoic acid was not improved by the co-oxidation of cis-cyclooctene. These results raised questions regarding the activated oxygen specie(s) responsible for the epoxidation and the exact mechanism of this reaction.
- This article is part of the themed collection: Catalysis in Flow Chemistry

 Please wait while we load your content...
Please wait while we load your content...