Stability, scalability, and reusability of a volume efficient biocatalytic system constructed on magnetic nanoparticles†‡
Abstract
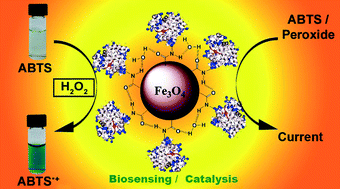
This report investigates for the first time stability, scalability, and reusability characteristics of a protein nano-bioreactor useful for green synthesis of fine chemicals in aqueous medium extracting maximum enzyme efficiency. Enzyme catalysts conjugated with magnetic nanomaterials allow easy product isolation after a reaction involving simple application of a magnetic field. In this study, we examined a biocatalytic system made of peroxidase-like myoglobin (Mb), as a model protein, to covalently conjugate with poly(acrylic acid) functionalized magnetic nanoparticles (MNPs, 100 nm hydrodynamic diameter) to examine the catalytic stability, scalability, and reusability features of this bioconjugate. Application of the conjugate was effective for electrochemical reduction of organic and inorganic peroxides, and for both peroxide-mediated and electrocatalytic oxidation of the protein substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) with greater turnover rates and product yields than Mb prepared in solution or MNP alone. Mb-attached MNPs displayed extensive catalytic stability even after 4 months of storage compared to Mb present in solution. Five- and ten-fold scale up of MNPs in the bioconjugates resulted in two- and four-fold increases in protein-catalyzed oxidation products, respectively. Nearly 40% of the initial product was present even after four reuses, which is advantageous for synthesizing sufficient products with a minimal investment of precious enzymes. Thus, the results obtained in this study are highly significant in guiding cost-effective development and efficient multiple uses of enzyme catalysts for biocatalytic, electrocatalytic, and biosensing applications via magnetic nanomaterials conjugation.

 Please wait while we load your content...
Please wait while we load your content...