The strongest CO binding and the highest C–O stretching frequency†
Abstract
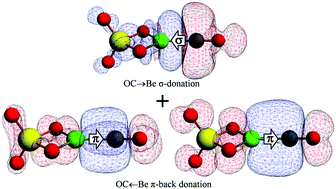
A coupled-cluster study is performed on CO bound BeY complexes (Y = O, CO3, SO4, NH, NCN, and NBO) to understand the effect of attached ligands (Y) on the CO binding ability and C–O stretching frequency (νCO). Herein, we report that BeNCN has the highest CO binding ability (via both C- and O-side binding) among the studied neutral Be-based clusters, whereas OCBeSO4 has the highest νCO among the neutral carbonyls. The nature and extent of shift in νCO compared to free CO are explained in terms of change in polarization in the bonding orbitals of CO and relative contribution from OC→BeY or CO→BeY σ-donation, and OC←BeY or CO←BeY π-back-donation. The largest blue-shift in OCBeSO4 and the largest red-shift in COBeNH are consequences of the smallest OC←BeSO4 π-back-donation and the largest CO←BeNH π-back-donation, respectively.


 Please wait while we load your content...
Please wait while we load your content...