Temperature and collision energy effects on dissociation of hydrochloric acid on water surfaces†
Abstract
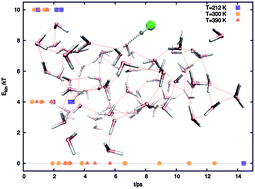
Collisions of HCl at the air–water interface modelled by a 72 molecule water slab are studied for a range of various impact energies and temperatures using ab initio molecular dynamics with density functional theory. A range of short-timescale events can follow the collision, from direct scattering to nondissociative trapping on the surface. In most cases, HCl dissociation occurs within a few picoseconds, followed by the formation of a solvent-separated ion pair, or rarely, the reformation of HCl. With increasing impact energy and/or system temperature, dissociation occurs more rapidly, with Cl− tending to diffuse deeper into the slab. At temperatures corresponding to the frozen water regime, dissociation is seen only once out of the five thermal collisions, but with the addition of a total of 4kT or more of kinetic energy to HCl, it occurs in all our trajectories within a few ps.

 Please wait while we load your content...
Please wait while we load your content...