Cyclisation of biscarbenoids – a novel mode of cyclobutadiene stabilisation†
Abstract
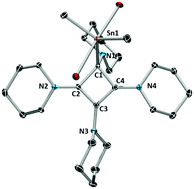
The reaction of the vicinal biscarbenoid Pip–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Pip with dimethyltin dichloride yields a unique tetraamino-substituted cyclobutadienyl system featuring a dative C–Sn interaction. DFT investigation of the reaction mechanism revealed that coordination of the stannyl fragment to the nucleophilic carbon leads to a metal-stabilised zwitterion, allowing for [2+2] cycloaddition under thermal conditions. The compound features a homoaromatic π-system comprising the three sp2-hybridised carbon atoms of the four-membered ring as a consequence of charge separation.
C–Pip with dimethyltin dichloride yields a unique tetraamino-substituted cyclobutadienyl system featuring a dative C–Sn interaction. DFT investigation of the reaction mechanism revealed that coordination of the stannyl fragment to the nucleophilic carbon leads to a metal-stabilised zwitterion, allowing for [2+2] cycloaddition under thermal conditions. The compound features a homoaromatic π-system comprising the three sp2-hybridised carbon atoms of the four-membered ring as a consequence of charge separation.

 Please wait while we load your content...
Please wait while we load your content...