Efficient access to alkynylated quinalizinones via the gold(i)-catalyzed aminoalkynylation of alkynes†
Abstract
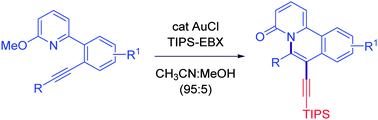
The gold-catalyzed aminoalkynylation of alkynes for the synthesis of quinalizinones is reported. For instance, the reaction of pyridinoalkynes with 1-[(triisopropylsilyl)-ethynyl]-1,2-benziodoxol-3(1H)-one (TIPS-EBX) in the presence of a catalytic amount of AuCl at 50 °C afforded alkynylated quinalizinones in 57–87% yields.

 Please wait while we load your content...
Please wait while we load your content...