Synergistic gold and enamine catalysis: intermolecular α-alkylation of aldehydes with allenamides†
Abstract
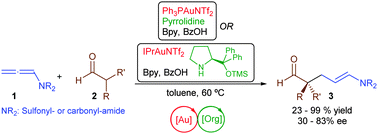
Aldehydes can be α-alkylated with allenamides by the combined action of an organocatalyst and a gold complex. The reaction requires the simultaneous generation of an enamine and a gold-activated allenamide. Importantly, by using a chiral amine as organocatalyst it is possible to obtain aldehyde products featuring all-carbon quaternary stereocenter at their α-position, with moderate to good levels of enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...